Carbon and water are the cornerstones of life on Earth. Many natural systems, biotic or abiotic, can persist because of these two very important materials. Almost all living organisms depend on and use both carbon and water for their growth and survival. Additionally, processes such as the carbon cycle and the water cycle make our planet hospitable to the proliferation of life.
To this day, water carbon climate plays a critical role in the survival of many species, humans included. In fact, because of the close correlation between water carbon climate and life on earth, research and conservation efforts worldwide are now directed to the protection of these priceless natural resources.
The Carbon Cycle
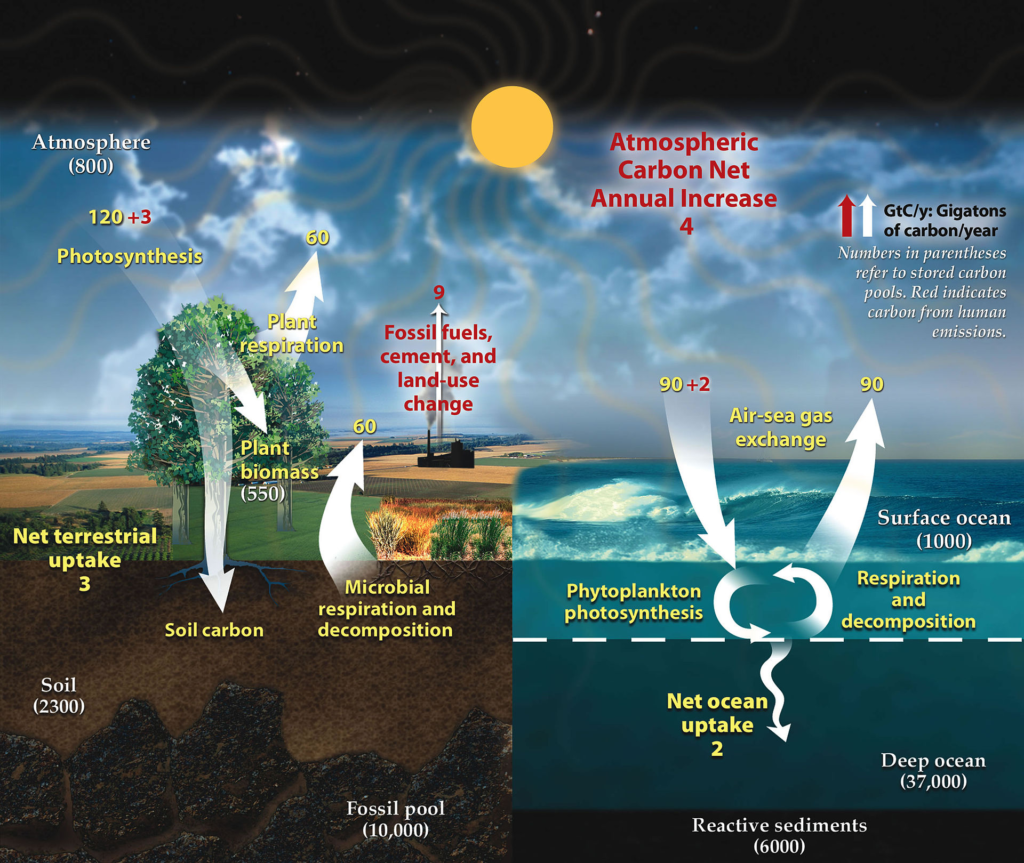
Carbon is the fourth most abundant element in our universe. The 65,600 billion metric tons of carbon stored in rocks accounts for a majority of the carbon on Earth. Other large carbon stores include the oceans, the atmosphere, the soil, plants, and fossil fuels.
Carbon is transferred from one store to another through the carbon cycle. In this manner, all carbon reserves on the planet are inextricably linked to one another. If a change in the cycle causes one store to lose carbon, more carbon will be kept in other stores. One notable example is how developments in human industry have taken large amounts of carbon from the Earth and put it in the atmosphere, increasing global temperatures.
Carbon is an element essential to all life on this planet. All forms of organic life are made up of carbon, consume carbon, and produce carbon. Human civilization is also built on carbon and the use of carbon. Our infrastructure, modes of transport, and technology all have carbon or utilise carbon in some way.
The Water Cycle
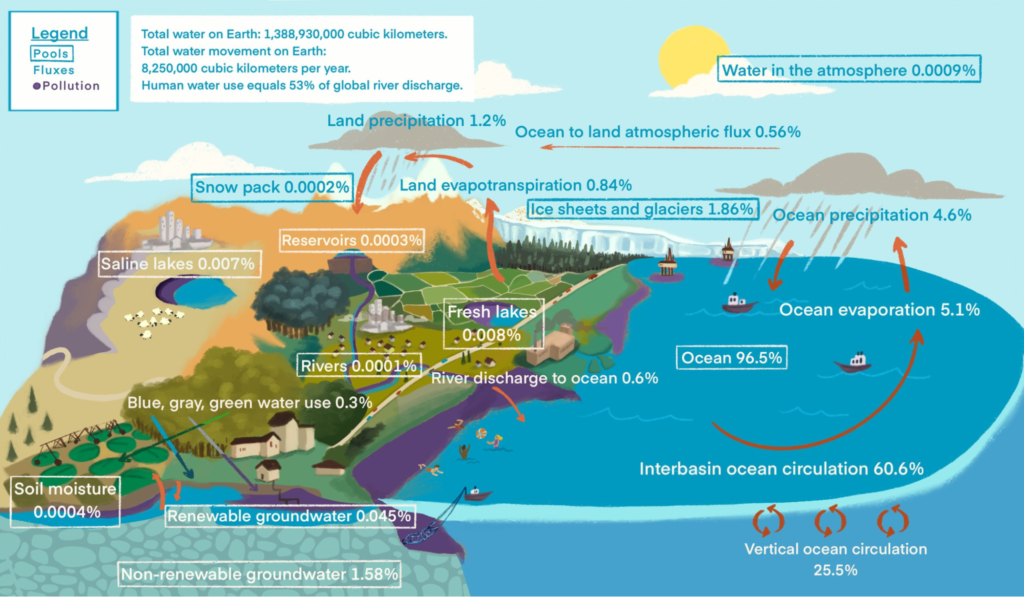
Water is one of the most abundant resources on Earth. On our planet, it exists in three states: solid ice, liquid water, and gaseous water vapour. The water cycle is the process in which water moves between the land, the ocean and the atmosphere. In this cycle, water evaporates from the Earth’s surface, ascends into the atmosphere, is cooled down and condenses in clouds, and falls in the form of precipitation.
Water that falls to the ground collects in bodies of water such as rivers, lakes, and oceans. Otherwise, it is absorbed into the soil or through porous layers of rock. Water from many different sources will again evaporate and rise into the atmosphere and continue in this perpetual cycle. The water cycle is a major proponent in creating various weather patterns on Earth.
Interactions of the Carbon and Water Cycles
Each in their own right, both the carbon cycle and the water cycle have great significance to the many biotic and abiotic processes that make life on Earth possible. However, it is important to know that these two cycles do not operate separately. Instead, they are in constant interaction with each other. A change in one system can inadvertently affect the other.
Interacting Processes
Photosynthesis and respiration
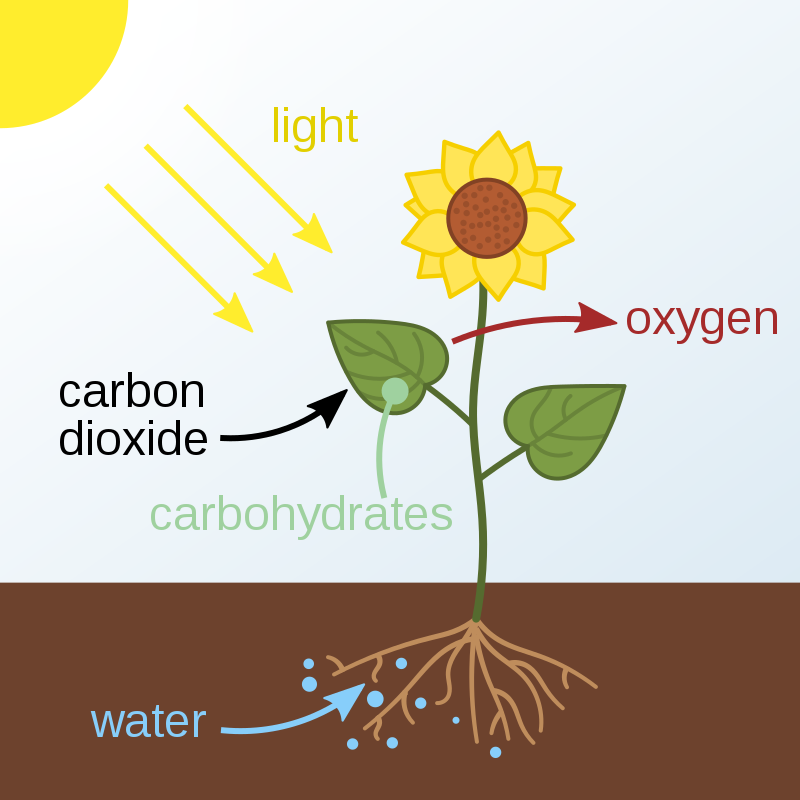
These two opposing processes cycle carbon and water between the atmosphere and the biosphere. Photosynthesis takes carbon dioxide from the atmosphere and water from the ground to create energy. On the other hand, organisms release carbon dioxide into the air as a byproduct of making energy through cellular respiration.
Weathering
When water runs across the land as a result of precipitation or run-off, it erodes the rock and soil that it passes. As a result of this weathering, carbon that is released from the lithosphere makes its way into the atmosphere and the oceans.

Exchange between the ocean and the atmosphere
Physical and biological pumps facilitate the exchange of carbon between the ocean and the atmosphere. Carbon dioxide from the atmosphere is dissolved in the ocean’s surface. In areas where surface waters sink due to their low temperature and higher density, physical pumps transport it to the deep oceans.
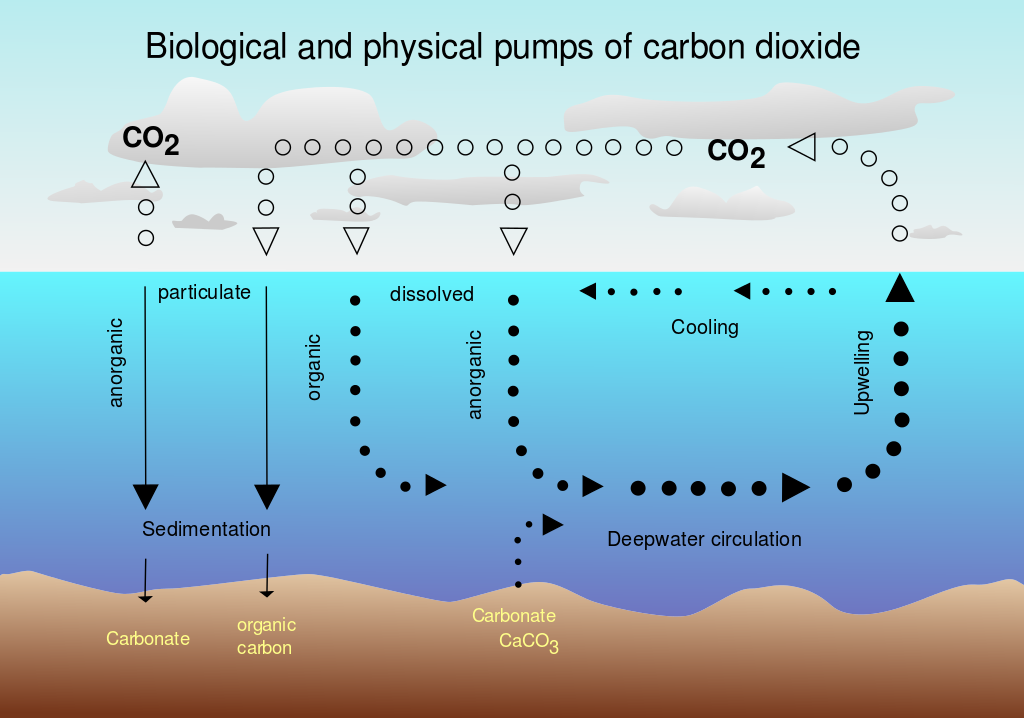
Thermohaline circulation
Around the world, thermohaline circulation carries heat energy and organic matter from one place to another. The movement of ocean currents also facilitates the physical pumps that take carbon from the atmosphere and into the depths of the oceans.
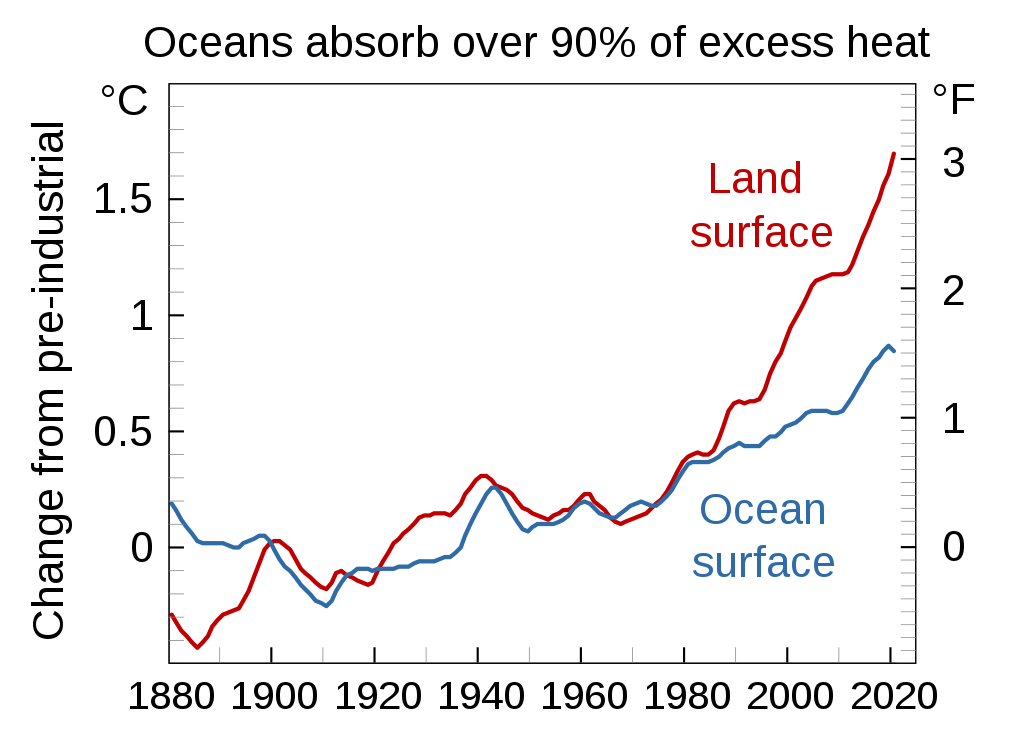
Ocean warming
In addition to the rise in atmospheric temperatures, excessive amounts of carbon in our atmosphere has also led to a rise in oceanic temperature as well. The increasingly pronounced impact of the greenhouse effect has led to the warming of oceans around the globe.
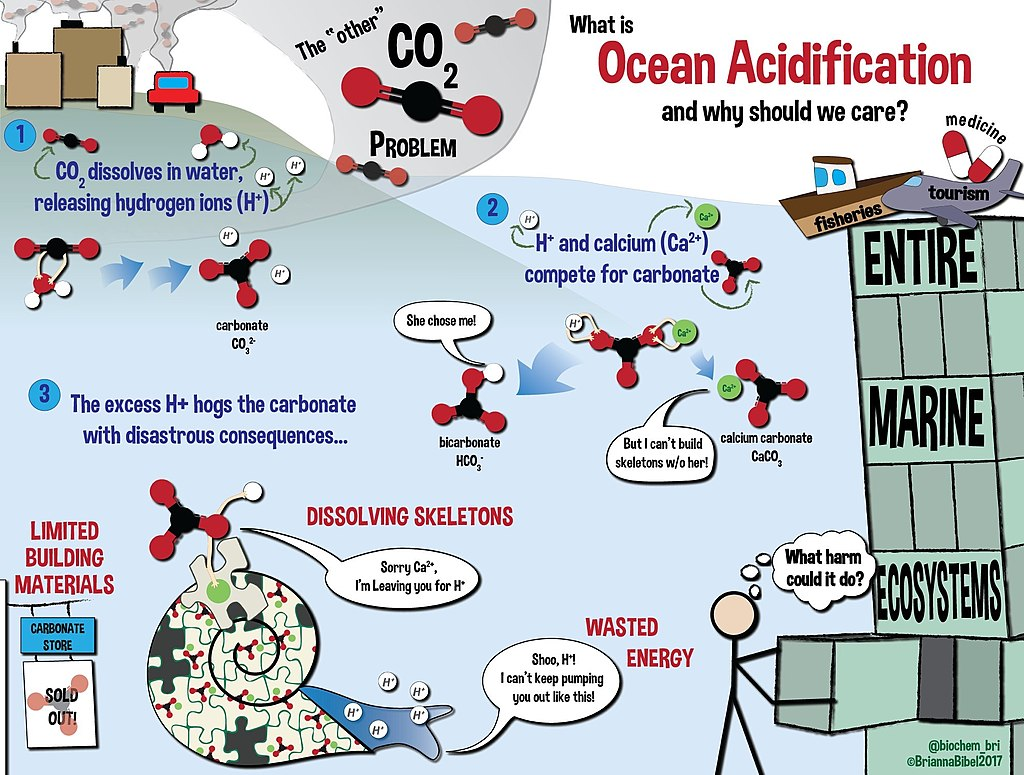
Ocean acidification
The increasing amount of carbon in our oceans has also led to an increase in their acidity. This can have significant detrimental effects on marine life, as many species can only survive in waters of a certain pH level.
Permafrost melting
The rise in global temperatures due to major changes in the carbon cycle also affects the cryosphere. The greenhouse effect has resulted in the melting of polar ice and permafrost. This also releases the carbon dioxide and methane trapped in the ice.

Volcanic outgassing
Volcanic eruptions lead to the melting of rock and the release of gasses trapped in the lithosphere into the atmosphere. Volcanic outgassing from both above ground and underwater sources can transfer carbon and water into the air above.
Climate Change
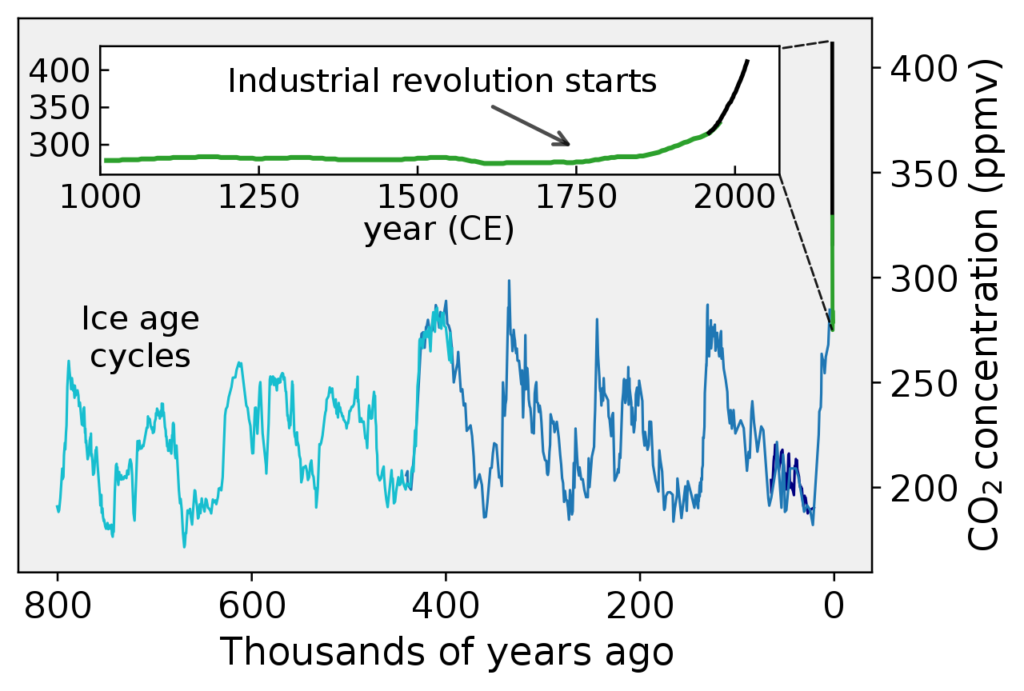
The Industrial Revolution marked the beginning of people burning fossil fuels for a variety of applications. Since then, there has been a steady increase in the amount of carbon dioxide that ends up in our atmosphere. Carbon dioxide concentrations have since then increased from 280 parts per million to 387 parts per million, a daunting 39% increase. This is the highest recorded concentration of atmospheric carbon dioxide in two million years.
On the other hand, methane concentrations in the year 1774 were at 715 parts per billion. In 2005, this number increased to 1774 parts per billion. This is the highest concentration of methane in the atmosphere in 650,000 years. In the span of three centuries, human activity has produced devastating impacts on the environment.
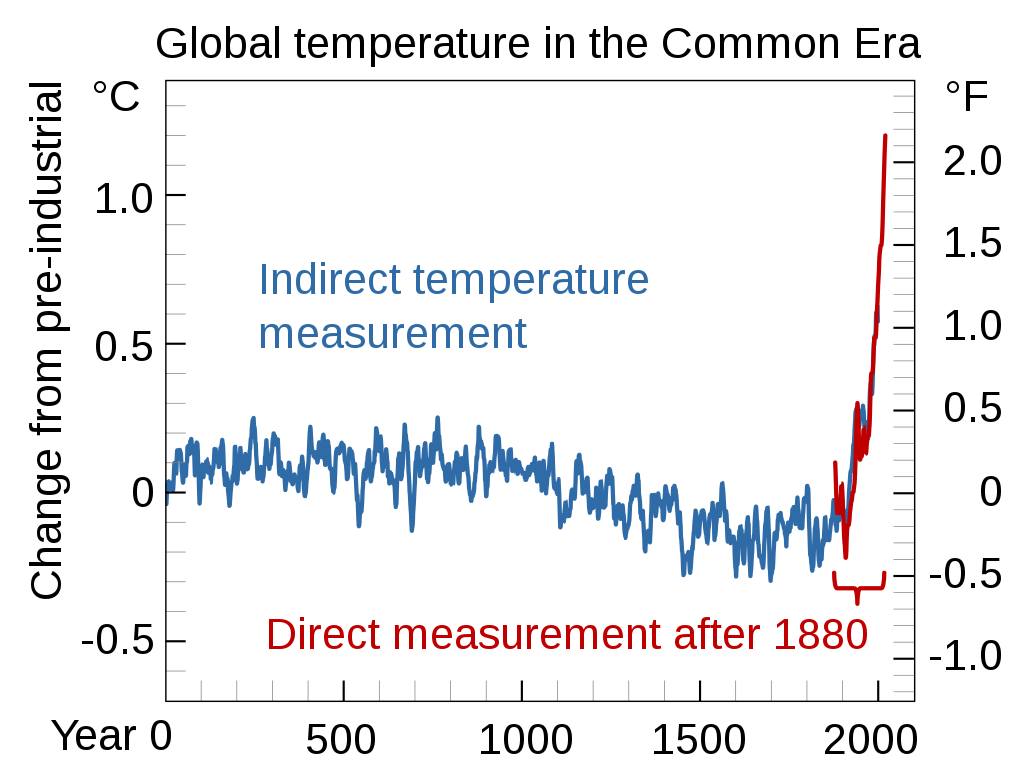
Effects on the Atmosphere
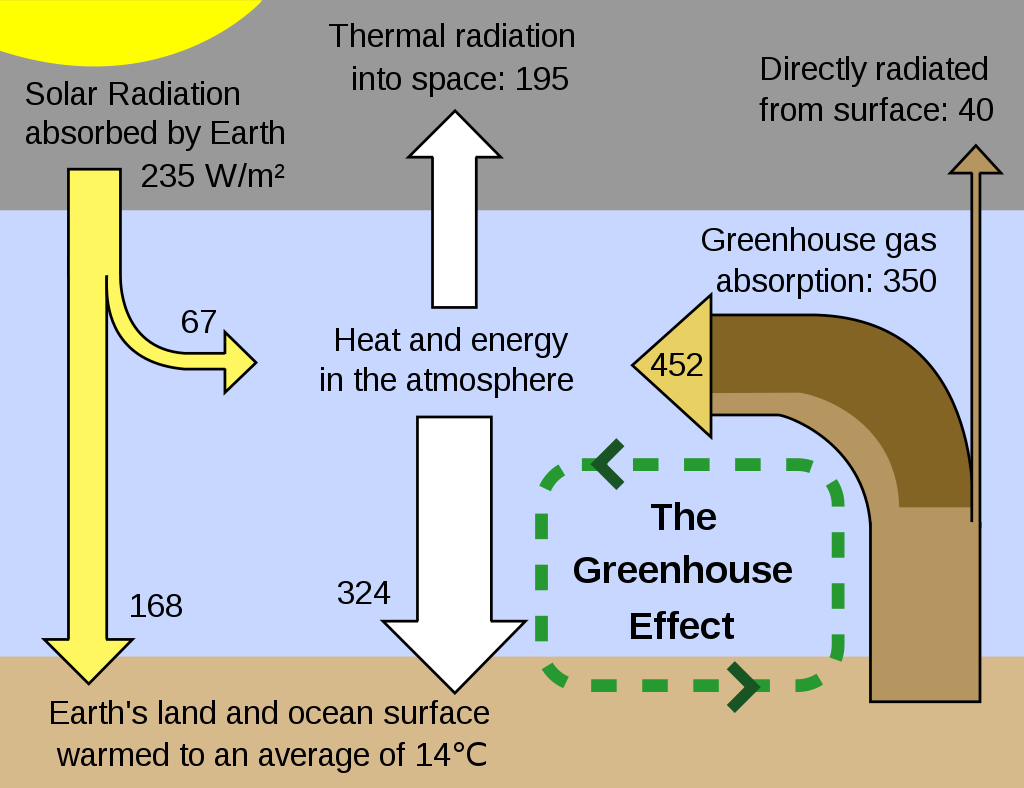
Scientists have determined carbon dioxide to cause around 20% of the greenhouse effect, water vapour for 50%, and clouds for 25%. The remaining amount is caused by aerosols and minor greenhouse gases such as methane.
It cannot be denied that the increase in the amount of carbon in the atmosphere has had significant effects on many sectors of the globe. Carbon dioxide (CO2) is the gas most responsible for controlling the Earth’s temperature. Greenhouse gases such as carbon dioxide, methane, and halocarbons absorb the energy emitted by the earth and re-emits it in a scatter of directions.
Some of the energy is sent back to the earth, heating the surface. Greenhouse gases help keep the Earth’s temperature to be hospitable to many life forms. However, too many greenhouse gases in the atmosphere lead to trapping too much of the planet’s heat, increasing global temperatures.
This has significant implications on the amount of water vapour in the atmosphere. The Earth’s temperature controls the amount of water vapour present in the air. Higher temperatures lead to higher evaporation rates, the expansion of air masses, and an increase in humidity. Cooler temperatures cause the condensation of water vapour, leading to its precipitation as rain, snow, or sleet.
Compared to water vapour, carbon dioxide may contribute less to the greenhouse effect; however, scientists have discovered it to be the gas responsible for setting the temperature. Carbon dioxide in the atmosphere is not as sensitive to changes in atmospheric temperature as water vapour and remains in its gaseous state for the most part. Higher amounts of carbon dioxide in the atmosphere lead to an increase in temperature, which means more water vapour in the air.
This increase in water vapour concentration further amplifies the greenhouse effect. On the other hand, a decrease in atmospheric carbon dioxide levels leads to a decrease in temperature, and the condensation and precipitation of water vapour. Thus, less carbon dioxide also means less water vapour and a reduction in the greenhouse effect.
The rise in carbon dioxide concentrations and greenhouse gases in our atmosphere has already led to an increase in global temperatures. Compared to temperatures recorded in 1880, average global temperatures have risen 0.8C. Due to the ocean’s absorption of heat, global warming caused by the greenhouse effect does not happen in an instant. It is projected that this value will rise by another 0.6 degrees celsius in the future.
Effects on the Ocean
Of the amount of carbon dioxide put in the atmosphere as a result of human activity, an estimate of 30% of it is diffused into the ocean through the direct chemical exchange. When carbon dioxide is dissolved in water, carbonic acid is produced. High amounts of atmospheric carbon dioxide translate into more carbonic acid in our oceans, increasing the water’s acidity. The ocean is usually slightly alkaline, however, this increase in carbonic acid has made waters a little less alkaline. Compared to pH levels in 1750, ocean surfaces today have dropped by 0.1 pH, a 30% change in acidity.
The change in the ocean’s pH level can affect marine life in mainly two ways. The first is the creation of bicarbonate from the reaction of carbonic acid with carbonate ions in the water. This has certain implications on shell-building organisms such as coral. The carbonate ions consumed in this reaction are what these organisms use to create their shells made out of calcium carbonate. When there is less carbonate available in the water, these organisms end up expending more energy than usual, and their shells come out thin and fragile.
The second way is when calcium carbonate is easier dissolved in more acidic waters. This change in acidity dissolves the calcium carbonate shells of marine organisms, making them weak. Over time, however, the acidic waters will dissolve rock easier and release more carbonate ions. This reaction will permit the ocean to better absorb more carbon dioxide.

Carbon dioxide also plays a critical role in the growth of marine plants and phytoplankton. It is possible for an increase in carbon dioxide to be beneficial for the growth of a few species of plants and phytoplankton that take carbon dioxide directly from the ocean. However, not all species can take advantage of this increase in carbon dioxide levels.
In contrast, the warming of oceans caused by the greenhouse effect may be detrimental to phytoplankton populations. Phytoplankton thrives in cool waters rich in nutrients. An increase in the ocean’s temperatures could limit the growth of these populations. In turn, this would lead to a decrease in an ocean’s ability to take in atmospheric carbon through photosynthesis and the fast carbon cycle.
Effects on Land
On land, plants and vegetation absorb around 25% of the carbon dioxide produced by human consumption and industry. Generally speaking, since the year 1960, there has been an increase in the carbon dioxide intake of plants. Although only a part of this increase was directly caused by fossil fuel emissions.
The increase in the amount of available atmospheric carbon has led to a phenomenon called carbon fertilisation. Since plants have more carbon dioxide they could use in photosynthesis, they experience increased growth rates. Scientific models predict that if the amount of carbon in the atmosphere was doubled, plants may grow in the range of 12–17% more than usual. However, these models do not necessarily reflect real-life scenarios, as plants do not just need carbon dioxide for growth.
To grow, plants need water, sunlight, and nutrients such as nitrogen. Carbon dioxide fertilisation only increases plant growth in as much as the plant’s ability to be provided for its other needs.
Climate change potentially has the largest impact on the land carbon cycle. The increasing amounts of carbon dioxide in the atmosphere leads to warmer temperatures. This increases humidity and extends the growing season. Although these have led to some increased growth in vegetation, plants may experience increased amounts of stress from the warmer weather.
In this new climate, plants would require more water to survive. As a result of water shortages and the increase in temperature, plants are now growing slower. Plants that lack water become dry and more susceptible to threats such as fire and insects.
Frequently Asked Questions
How is water essential for life on Earth?
Water is essential for life on Earth because it is involved in numerous biological processes. It serves as a universal solvent, enabling chemical reactions within cells. Water is also crucial for maintaining temperature regulation, facilitating nutrient transport, supporting plant photosynthesis, and providing habitats for various organisms.
How does the carbon cycle impact climate on Earth?
The carbon cycle plays a significant role in regulating Earth’s climate. Carbon dioxide (CO2) is a greenhouse gas that traps heat in the atmosphere, contributing to the natural greenhouse effect. Human activities, such as the burning of fossil fuels and deforestation, have increased CO2 concentrations, leading to enhanced greenhouse warming and climate change.
How does climate change affect life on Earth?
Climate change can have profound effects on life on Earth. Rising temperatures, shifting precipitation patterns, and extreme weather events can disrupt ecosystems, alter habitat availability, and impact biodiversity. Climate change can also affect the timing of seasonal events, such as flowering and migration, which can disrupt ecological relationships and threaten the survival of many species.
How do living organisms contribute to the carbon cycle?
Living organisms play a crucial role in the carbon cycle. Through the process of photosynthesis, plants and algae absorb carbon dioxide from the atmosphere and convert it into organic compounds, releasing oxygen as a byproduct. Carbon is then transferred through the food chain as organisms consume plants or other organisms. When organisms respire or decompose, carbon dioxide is released back into the atmosphere, completing the cycle.
How do changes in water availability affect ecosystems and human societies?
Changes in water availability can have significant impacts on ecosystems and human societies. Droughts can lead to water scarcity, affecting agricultural productivity, disrupting ecosystems, and causing water-related conflicts. Increased flooding can result in property damage, displacement of populations, and contamination of water sources. Changes in water availability also influence the distribution of species, affect water-dependent industries, and can impact overall economic and social well-being.
References
Carbon & water cycles – Life on Earth. (n.d.). Retrieved from CoolGeography: http://www.coolgeography.co.uk/advanced/Carbon_water_cycles_Life_Earth.php
The Carbon Cycle. (n.d.). Retrieved from Nasa Earth Observatory: https://earthobservatory.nasa.gov/features/CarbonCycleThe Water Cycle. (n.d.). Retrieved from Global Precipitation Measurement: https://gpm.nasa.gov/education/water-cycle